INTRODUCTION
In the past decade, diagnostic strategies and management modalities for male infertility have undergone extensive modifications, making male infertility one of the fastest growing fields among all subspecialties in urology. The substantially increased success in the management of male factor infertility witnessed in recent years is attributable to improved techniques in microsurgical reconstruction for obstruction,1, 2, 3, 4 varicocelectomy for enhancement of spermatogenesis,5, 6 and refined surgical techniques for sperm retrieval7 combined with the successful application of in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI).
Even men with testicular failure (now called nonobstructive azoospermia), once regarded as hopeless cases, can now father biological offspring.8
The development of high-resolution scrotal and transrectal ultrasound has substantially improved diagnostic capabilities. Ultrasound-guided retrieval of seminal vesicle sperm not only provides diagnostic information but also allows retrieval of sperm for IVF with ICSI. Similarly, whereas testicular biopsy to confirm the presence of spermatogenesis was generally reserved for men with normal follicle-stimulating hormone (FSH) levels and testicular volumes, the procedure is now indicated in all men with obstructive or nonobstructive azoospermia.
Furthermore, testicular biopsy is now also a therapeutic tool for retrieval of sperm that may be used immediately for IVF with ICSI or cryopreserved for future use.
Although IVF with ICSI now provides the means to treat even the most severe forms of male-factor infertility (including irreparable reproductive tract obstruction and nonobstructive azoospermia), the procedure is costly and assumes the risks associated with ovarian hyperstimulation, oocyte retrieval, and multiple gestations. Moreover, because it bypasses all natural biological barriers, ICSI raises realistic concerns about passing genetic abnormalities to offspring.9, 10
Recent analyses clearly indicate that specific treatments for male-factor infertility (microsurgical reconstruction for obstructive azoospermia and varicocelectomy) remain the safest and most cost-effective ways of managing infertile men, when applicable.11, 12, 13, 14 Thus, proper selection of couples for assisted reproductive technologies and careful genetic counseling is essential.
For men with irreparable obstructions and nonobstructive azoospermia caused by testicular failure, surgical sperm retrieval, and assisted fertilization via IVF/ICSI are feasible management options. The development and recent refinements in various techniques for surgical sperm retrieval from testes, epididymides, or seminal vesicles using percutaneous or open surgical approaches have expanded treatment options for infertile men.
In particular, use of the operating microscope to identify the individual seminiferous tubules more likely to contain sperm has improved the success of testicular sperm extraction15 and minimized associated morbidity.16
Microsurgical techniques also have been extended to varicocelectomy. Varicoceles, long known to be associated with male infertility, have now clearly been shown to result in progressive duration-dependent testicular injury.17, 18, 19, 20, 21, 22, 23, 24 Accordingly, the indications for surgery have been expanded in recent years.
Microsurgical varicocelectomy, previously reserved only for men with oligospermia, has now been applied to men with nonobstructive azoospermia, resulting in a return of spermatogenesis and sperm to the ejaculate in more than 50% of cases.5, 25 In addition, whereas surgical varicocele repair was previously reserved for already infertile men, earlier intervention now offers the means to not only salvage remaining testicular function but also to prevent future infertility. Given the now substantial evidence that varicocele adversely affects Leydig cell function and results in serum testosterone levels lower than are observed in age-matched controls without varicocele, varicocelectomy might be expected to halt and even partially reverse an otherwise inevitable decline.26
In selected men, varicocelectomy may be an effective treatment for symptomatic age-related androgen deficiency, a condition that has been dubbed andropause.
Surgical treatment of male infertility and scrotal disorders poses little risk to patients while offering the promise of new life and improved quality of life for infertile couples. The techniques described in this chapter can be technically demanding and require both intensive microsurgical training and a thorough knowledge of the anatomy and physiology of the male reproductive system.
INDICATIONS FOR TESTIS BIOPSY
Testis biopsy is indicated in azoospermic men with testis of normal size and consistency, palpable vasa deferentia, and normal serum FSH levels. Under these circumstances, biopsy will distinguish obstructive azoospermia from primary seminiferous tubular failure. In the testes of men presenting with congenital absence of vasa, biopsy always reveals normal or at least some spermatogenesis,27 and biopsy is not necessary before definitive sperm aspiration and IVF with ICSI.
The ability to achieve pregnancy with only a single testicular sperm has broadened the indications for testis biopsy to virtually all azoospermic men, and has turned biopsy into a potentially therapeutic, as well as diagnostic procedure. Even men with markedly elevated serum FSH levels and small soft testes, in whom testicular failure is certain, often harbor rare mature sperm in their testes. These sperm can be extracted using techniques described later in this chapter and used for IVF with intracytoplasmic injection of testicular sperm.
The heterogeneity of the testes of men with nonobstructive azoospermia coupled with the ability of testicular sperm to acquire motility,28 has prompted changes in the techniques of testis biopsy. Examination of fresh unfixed tissue for the presence of sperm with tails and possible motility, and examination of multiple samples, if sperm are not found initially, is now mandatory. Furthermore, optimal care requires the availability, at the time of biopsy, of an andrology laboratory capable of processing and cryopreserving any sperm found at the time of biopsy.
Diagnostic biopsy usually should be performed bilaterally regardless of the size discrepancy between the two testes. Good spermatogenesis is sometimes found in small firm testes while biopsies of large healthy testes may reveal maturation arrest.
Open Testis Biopsy – Microsurgical Technique
Open biopsy remains the gold standard as it yields sufficient tissue both for accurate diagnosis and for recovery of sperm for IVF.15, 16, 29 Open testis biopsy may be performed using general, spinal, or local anesthetic. Local anesthesia with spermatic cord block can be effective. However, in animal studies, the incidence of accidental damage to the testicular artery during blind cord block is 5%.30
Local anesthesia of just the skin and tunicas without a cord block is uncomfortable. If there has been previous scrotal surgery with scar or adhesions and more extensive dissection and manipulation may be required, general or spinal anesthetic is preferred.
When performing testis biopsy, the surgeon must provide an optimal tissue sample, avoid trauma to the specimen, and avoid injury to the epididymis and testicular blood supply. Open biopsy under magnification (preferably with an operating microscope) satisfies these requirements.
Bilateral 1-cm transverse scrotal incisions provide good exposure with a minimum of scrotal skin bleeding (Fig. 1).
Alternatively, a single vertical incision in the median raphe may be employed. Use of loupes or an operating microscope allows ready identification of a relatively avascular area on the tunica albuginia where an incision may be made.
A 3–4 mm incision is made in the tunica albuginea with a 15-degree microknife (Fig. 2).
A pea-size sample of seminiferous tubules is excised with razor-sharp iris scissors (Fig. 3).
When handling testis biopsy material for permanent fixation, it is important to avoid tissue traumatization by forceps handling in any way as this may distort the testicular architecture.
|
|
|
|
|
|
The specimen is then deposited directly into Bouins, Zenkers, or collidine-buffered glutaraldehyde solution. Formalin fixation results in distortion of testicular histology and should not be used for testis biopsy. A touch-prep is made by blotting the cut surface of the testis several times with a glass slide (Fig. 4), and adding a drop of saline or lactated Ringer’s and a cover slip.
Examination under high power using a light microscope with or without phase contrast will reveal the presence of sperm with tails and allow assessment of motility (Fig. 5). If no sperm are found on the touch prep, a second specimen may be cut for a wet squash prep (Fig. 6). In this case, the specimen is placed on a slide, a drop of saline is added, and the specimen is crushed under a coverslip.28 If no sperm are found, the tunica is closed with two to three interrupted sutures of 6-0 nylon (Fig. 7) and another area undergoes biopsy through the same skin incision.
As described later in this chapter, use of an operating microscope providing 10–25 times magnification may allow selective biopsy of larger seminiferous tubules more likely to contain sperm.15
|
|
|
|
|
|
|
|
If sperm are identified, the slide and additional tissue removed is sent for cryopreservation by the andrology laboratory. The location of the biopsy site and whether sperm were found at that site is noted in the operation report and the patient’s chart. This facilitates identification of the sperm-bearing site for future IVF/ICSI with testicular extraction. The tunica albuginea is closed with two to three interrupted sutures of 6-0 nylon. Nonabsorbable sutures are used to aid in identifying the biopsy site at the time of future testicular sperm retrieval.
Percutaneous Testis Biopsy
Percutaneous testis biopsy using the same 14-gauge biopsy gun used for prostatic biopsy is a blind procedure and could result in unintentional injury to either the epididymis or the testicular artery. This technique should not be used when previous surgery has resulted in scarring and obliteration of normal anatomy. Diagnostic specimens obtained in this way often contain few tubules with poorly preserved architecture. When performed under local anesthesia, a cord block is necessary to minimize pain.
The technique of percutaneous biopsy is further illustrated later. As a therapeutic tool for sperm retrieval, percutaneous gun biopsy is most useful for fresh sperm retrieval for IVF/ICSI in men with obstructive azoospermia and normal spermatogenesis.
Percutaneous Testicular Aspiration
Testicular aspiration performed with a 23-gauge needle is probably less invasive and less painful than percutaneous biopsy. Although flow cytometric evaluation of this material can distinguish haploid from diploid cells and therefore confirm the presence or absence of late stages of spermatogenesis,31 direct wet examination of the aspirate for sperm and assessment of motility provide the most practical clinical information.
Three or four aspirations can be performed until sperm are identified. In cases of obstructive azoospermia, these sperm can be used for IVF with ICSI32 when sperm could not be retrieved from the epididymis.
Complications of Testis Biopsy
Carefully performed, testis biopsy is associated with few complications. The most serious complication associated with testis biopsy is inadvertent biopsy of the epididymis. If histological evaluation of the biopsy material reveals epididymis with sperm within the epididymal tubule, obstruction of the epididymis at the site of the biopsy is certain.
If, however, there are no sperm within the epididymal tubules, the patient either is obstructed above the level of the epididymal biopsy or has primary seminiferous tubular failure and no harm has been done.
The most common complication of testis biopsy is hematoma. Use of magnification to avoid vessels and a bipolar cautery for hemostasis will help prevent this complication.16 Proper closure of the well-vascularized tunica vaginalis with a continuous suture of 5-0 Vicryl will minimize bleeding and adhesions. With the rich blood supply of the scrotum and its contents, wound infection is rare in the absence of hematoma and antibiotics are unnecessary.
VASOVASOSTOMY
The number of American men who undergo vasectomy has remained stable at approximately 500,000 per year, as has the divorce rate of 50%. Surveys suggest that 2–6% of vasectomized men will ultimately seek reversal. Furthermore, obstructive azoospermia can be the result of iatrogenic injuries to the vas deferens, usually from hernia repair, in 6% of azoospermic men.33
Preoperative Evaluation
Before attempted surgical reconstruction of the reproductive tract, spermatogenesis in the patient should be evident. A previous history of natural fertility prevasectomy is usually adequate. In other cases, a testicular biopsy may be indicated to confirm the presence of spermatogenesis.3
Physical Examination
Physical examination includes the following:
| Testis | Small or soft testes suggest impaired spermatogenesis and predict a poor outcome |
| Epididymis | An indurated irregular epididymis often predicts secondary epididymal obstruction, necessitating vasoepididymostomy |
| Hydrocele | The presence of a hydrocele in face of excurrent ductal system obstruction is often associated with secondary epididymal obstruction. Surgeons attempting reconstruction should be aware of the possibility of a vasoepididymostomy being required |
| Sperm granuloma | A sperm granuloma at the testicular end of the vas suggests that sperm have been leaking at the vasectomy site. This vents the high pressures away from the epididymis and is associated with a better prognosis for restored fertility regardless of the time interval since vasectomy |
| Vasal gap | When a very destructive vasectomy has been performed, most of the scrotal straight vas may be absent or fibrotic and the patient should be advised that inguinal extension of the scrotal incision will be necessary to mobilize adequate length of vas to enable a tension-free anastomosis |
| Scars from previous surgery | Operative scars in the inguinal or scrotal region should alert the surgeon to the possibility of iatrogenic vasal or epididymal obstruction |
Laboratory Tests
Semen analysis with centrifugation and examination of the pellet for sperm should be performed preoperatively. Complete sperm with tails are found in 10% of preoperative pellets a mean of 10 years after vasectomy.34 Under these circumstances, sperm are certain to be found in the vas on at least one side, indicating a favorable prognosis for restored fertility. Men with a low semen volume should have a transrectal ultrasound to alert one to the possibility of an additional ejaculatory duct obstruction.
In serum and antisperm antibody studies, the presence of serum antisperm antibodies corroborates the diagnosis of obstruction and the presence of active spermatogenesis. At present, this test is of unknown prognostic value and is optional. For serum FSH, men with small soft testes should have serum FSH measured. An elevated FSH predicts impaired spermatogenesis and a poorer prognosis. All vasectomy reversal candidates older than age 40 should have serum prostate-specific antigen (PSA) measured.
Anesthesia
Light general anesthesia is preferred. Slight movements are greatly magnified by the operating microscope and disturb performance of the anastomosis. In patients who have a palpable sperm granuloma, whose vasal ends are easily palpable and the time since vasectomy is short, the likelihood of a secondary epididymal obstruction requiring a vasoepididymostomy, which is a more complicated surgery than vasovasostomy, is low.
Thus, if these patients are cooperative, a regional or even local anesthesia with sedation can be used for vasectomy reversal. When large vasal gaps are present, extensions of the incisions high into the inguinal canal may be necessary. Furthermore, if vasoepididymostomy is necessary, the operating time could exceed 4 or 5 hours. Local anesthesia limits the options available to the surgeon. Hypobaric spinal anesthesia with long acting agents such as Marcaine can provide 4–5 hours of anesthesia time and have the advantage of eliminating lower body motion. Epidural anesthesia with an indwelling catheter can be equally effective.
Incision
Bilateral high-vertical scrotal incisions provide the most direct access to the obstructed site in cases of vasectomy reversal. Length is usually a problem on the abdominal end but not on the testicular end. Mark the location of the external inguinal ring (Fig. 8). If the vasal gap is large, or the vasectomy site is high, this incision can easily be extended inguinally toward the external ring.
If the vasectomy site is low, it is easy to pull up the testicular end. This incision should be made at least 1 cm lateral to the base of the penis. The testis should be delivered with the tunica vaginalis left intact. This provides excellent exposure of the entire scrotal vas deferens and, if necessary, the epididymis.
|
|
Preparation of the Vasa
The vas is mobilized enough to allow a tension-free anastomosis. To preserve good blood supply the vas should not be stripped of its sheath. The obstructed segment and, if present, sperm granuloma at the vasectomy site should be dissected out and excised.
When the vasal gap is extremely large, additional length can be achieved by dissecting the entire convoluted vas free of its attachments to the epididymal tunica, (Fig. 9) allowing the testis to drop upside down. These maneuvers can provide an additional 4–6 cm of length.
|
|
After the vasa have been freed, the testicular end of the vas is cut tranversely (Fig. 10). The cut surface of the testicular end of the vas deferens is inspected using 15–25 power magnification. A healthy white mucosal ring should be seen, which springs back immediately after gentle dilation. The muscularis should appear smooth and soft. A gritty-looking muscularis layer indicates the presence of scar/fibrotic tissues.
|
|
Once a patent lumen has been established on the testicular end, the vas is milked and a clean glass slide is touched to its surface. The vasal fluid is immediately mixed with a drop or two of saline or Ringer’s lactate and preserved under a cover slip for microscope examination. The abdominal end of the vas deferens is prepared in a similar manner and the lumen gently dilated with a microvessel dilator and cannulated with a 24-gauge angiocatheter sheath. Injection of saline or Ringer’s lactate confirms its patency.
When to Perform Vasoepididymostomy
The gross appearance of fluid expressed from the testicular end of the vas is usually predictive of findings on microscopic examination (Table 1). If microscopic examination of the vasal fluid reveals the presence of sperm with tails, vasovasostomy is performed.
If no fluid is found, a 24-gauge angiocatheter sheath is inserted into the lumen of the testicular end of the vas and barbotaged with 0.1-mL of saline while the convoluted vas is vigorously milked. The barbotage fluid is expressed onto a slide and examined. Men with large sperm granulomas often have virtually no dilation of the testicular end of the vas and little or no fluid initially; however, with barbotage and vigorous milking, invariably sperm can be found in this scant fluid. If there is no sperm granuloma, and the vas is absolutely dry and spermless after multiple samples are examined, vasoepididymostomy is indicated.
If the fluid expressed from the vas is found to be thick, white, water-insoluble, and toothpaste-like in quality, microscope examination rarely reveals sperm. Under these circumstances, the tunica vaginalis is opened and the epididymis inspected. If clear evidence of obstruction is found, i.e. an epididymal sperm granuloma with dilated tubules above and collapsed tubules below, vasoepididymostomy is performed.
When in doubt, or if not very experienced with vasoepididymostomy, vasovasostomy should be performed. However, only 15% of men with bilateral absence of sperm in the vasal fluid after barbotage and an intensive search will have sperm return to the ejaculate after vasovasostomy.35
Table 1. Surgical techniques for sperm retrieval
| Advantage | Disadvantages | |
| MESA (microsurgical epididymal sperm aspiration) | Microsurgical procedure allows lower complication rate | Requires anesthesia and microsurgical skills |
| Epididymal sperm has better motility than testicular sperm | Not indicated for nonobstructive azoospermia | |
| Large number of sperm can be harvested for cryopreservation of multiple vials in a single procedure | ||
| PESA (percutaneous epididymal sperm aspiration) | No microsurgical skill required | Complications include hematoma, pain, and vascular injury to testes and epididymis |
| Local anesthesia | Variable success in obtaining sperm | |
| Epididymal sperm has better motility than testicular sperm | Smaller quantity of sperm obtained than with MESA | |
| Not indicated in nonobstructive azoospermia | ||
| TESA (testicular sperm aspiration) | No microsurgical skill required | Immature or immotile testicular sperm |
| Local anesthesia | Small quantity of sperm obtained | |
| Can be used for obstructive azoospermia | Poor results in nonobstructive azoospermia | |
| Complications include hematoma, pain, and vascular injury to testes and epididymis | ||
| TESE (testicular sperm extraction) | Low complication rate if performed microsurgically | Requires anesthesia and microsurgical skills |
| Preferred technique for nonobstructive azoospermia |
When copious, crystal clear, water-like fluid squirts out from the vas and no sperm are found in this fluid, a vasovasostomy is performed because the likelihood is that sperm will return to the ejaculate after a vasovasotomy is performed.
Anastomotic Techniques: Keys to Success
All successful vasovasostomy techniques depend on adherence to surgical principles that are universally applicable to anastomoses of all tubular structures. These include:
| 1 | Accurate mucosa-to-mucosa approximation | In human vasovasostomy, the lumen on the testicular side is usually dilated, often to diameters 2–5 times that of the abdominal side. Techniques that work well with lumina of equal diameters may be less successful when applied to lumina of markedly discrepant diameters |
| 2 | Leakproof anastomosis | Sperm are highly antigenic and provoke an inflammatory reaction when they escape from the normally intact lining of the excurrent ducts of the male reproductive tract. Extravasated sperm adversely influence the success of vasovasostomy.36 Unlike blood vessel anastomoses, in which platelets and clotting factors seal the gaps between sutures, vasal and epididymal fluid contain no platelets or clotting factors, so the water-tightness of the anastomosis is entirely dependent on the mucosal sutures |
| 3 | Tension-free anastomosis | When an anastomosis is performed under tension, sperm may appear in the ejaculate for several months after surgery. Ultimately, sperm counts and motility will decrease and azoospermia may ensue. At re-exploration, only a thin fibrotic band is found at the anastomotic site. This can be prevented by adequately freeing up the vasa and placement of re-enforcing sutures in the sheath of the vas |
| 4 | Good blood supply | If the cut vas exhibits poor blood supply it should be recut until healthy bleeding is encountered. If extensive resection is necessary, additional length should be obtained using the techniques previously described |
| 5 | Healthy mucosa and muscularis | If the mucosa or cut surface of the vas exhibits poor distensibility after dilation, peels away from the underlying muscularis, or shreds easily, then the vas should be cut back until healthy mucosa is found. Surgeons should be aware that if a needle electrocautery was used in vasectomy, the area of damage to the mucosa and muscularis by the electric current may extend far beyond the tip of the needle cautery. If the muscularis is found to be fibrotic or gritty, the vas must be recut until healthy tissue is found |
| 6 | Good atraumatic anastomotic technique | If multiple surgical errors occur during the procedure, such as inadvertent cutting of the mucosa with the needles when placing sutures, tearing through of sutures, or backwalling of the mucosa, the anastomosis should be resected and reperformed immediately. |
Microsurgical Multilayer Microdot Method
The microdot technique was developed to properly handle markedly discrepant luminal diameters of the two ends of the vasa in performing vasovasostomy. This technique ensures precise suture placement by exact mapping of each planned suture. The microdot method separates the planning from the placement.37 This allows focus on only one task at a time and results in substantially improved accuracy.
A microtip marking pen (Devon Skin Marker Extra Fine #151; 1-800-DEVON PO) is used to map out planned needle exit points (Fig. 11). Exactly six monofilament 10-0 double-armed nylon mucosal sutures (first layer) are used for every anastomosis (Fig. 12) because they are easy to map out and always result in a leakproof closure even when the lumen diameters are markedly discrepant.
|
|
|
|
After completion of the mucosal layer (Fig. 13), six 9-0 deep muscularis sutures (second layer) are placed exactly in between each mucosal suture, just above, but not penetrating, the mucosa (Figs. 14, 15, and 16). Six additional 9-0 nylon interrupted sutures are then placed between each muscular suture (Fig. 17). This third layer of closure is performed purely on the adventitial layer that covers the underlying mucosal suture. The anastomosis is finished by approximating the vasal sheath with six interrupted sutures of 7-0 PDS (fourth layer) completely covering the anastomosis and relieving it of all tension (Fig. 18). All anastomosis consists of four layers, six sutures per layer, for a total of 24 sutures.
|
|
|
|
|
|
|
|
|
|
|
|
The dartos layer is approximated with interrupted 4-0 absorbable sutures and the skin with subcuticular sutures of 5-0 Monocryl. The wound heals with a fine scar. Virtually all of our procedures are performed on an ambulatory basis.
Postoperative Issues
Sterile fluffs gauze dressings are held in place with a snug-fitting scrotal supporter. Only perioperative antibiotics are used. Patients are discharged with a prescription for acetaminophen with codeine. They shower 48 hours after surgery. They wear a scrotal supporter at all times (except in the shower), even when sleeping, for 6 weeks postoperatively.
Thereafter, a scrotal supporter is worn during athletic activity until pregnancy is achieved. Deskwork is resumed in 3 days. No heavy work or sports are allowed for 3 weeks. No intercourse or ejaculation are allowed for 4 weeks postoperatively. Semen analyses are obtained at 1, 3, and 6 months postoperatively and every 6 months thereafter. If azoospermia persists at 6 months, another vasovasostomy or vasoepididymostomy will be necessary.
Postoperative Complications
The most common complication is hematoma. In 2100 operations seven small hematomas occurred. None required surgical drainage. Most were walnut-sized and perivasal. They take 6–12 weeks to resolve. Wound infection has not occurred. Late complications include sperm granuloma at the anastomotic site (approximately 5%). This usually is a harbinger of eventual obstruction. Late stricture and obstruction are disappointingly common.
Progressive loss of motility followed by decreasing counts indicates stricture. Our recent switch back to nylon sutures,38 use of the microdot system to prevent leaks, extensive dissection of the vas until healthy mucosa and muscularis and a good blood supply are reached, as well as generous use of scrotal support until pregnancy is established, may reduce this incidence. Because of the significant rate of late stricture and obstruction, we strongly encourage cryopreservation of semen specimens as soon as motile sperm appear in the ejaculate.
Long-Term Follow-Up Evaluation After Vasovasostomy
When sperm are found in the vasal fluid on at least one side at the time of surgery, the anastomotic technique described results in the appearance of sperm in the ejaculate in 99.5% of men.37 Late obstruction, after initial patency, may occur in up to 12% of men by 14 months postoperatively.1 Pregnancy has occurred in 52% of couples followed-up for at least 2 years, and 63% when female factors are excluded.
SURGERY OF EPIDIDYMIS
Detailed knowledge of epididymal anatomy and physiology is essential before undertaking surgery of this delicate but important structure. Sperm motility and fertilizing capacity progressively increase during passage through the 200 µm diameter, 12–15 foot long, tightly coiled, single tubule. When the epididymis is obstructed and functionally shortened after vasoepididymostomy, even very short lengths of epididymis are able to adapt and allow some sperm to acquire motility and fertilizing capacity.28, 37
Adaptation may gradually continue up to 2 years after surgical reconstruction, with progressive improvement in the fertility and motility of sperm. Nevertheless, preservation of the greatest possible length of functional epididymis is most likely to result in the best sperm quality after vasoepididymostomy.39, 40
Furthermore, because the wall of the epididymis is thinnest in the caput region and gradually thickens, caused by the increasing numbers of smooth muscle cells in its more distal (inferior) end, anastomoses are technically easier to perform and more likely to succeed in its distal regions. Because the corpus and cauda epididymis is a single tubule with a very small diameter, injury or occlusion of a tubule anywhere along its length will lead to total obstruction of outflow at that level. For these reasons, magnification, with loupes for macrodissection and with the operating microscope for anastomosis, is essential for performing all epididymal surgery.
Fortunately, the epididymis is blessed with a rich blood supply derived from the testicular vessels superiorly and the deferential vessels inferiorly. Because of the extensive interconnections between these branches, either the testicular or deferential branches (but not both) to the epididymis may be divided without compromising epididymal viability.
Conversely, because the epididymal branches of the testicular artery are medial to and separate from the main testicular artery and veins, surgical procedures may be performed on the epididymis without compromise to testicular blood supply.
Spermatocelectomy
A spermatocele is the epididymal equivalent of a berry aneurysm; they may occur anywhere in the epididymis but are more common in the caput region. They are exceedingly common, increasing in frequency with age, and identified incidentally in up to 30% of men undergoing high-resolution scrotal ultrasonography. Intervention for spermatocele is rarely indicated. Spermatoceles are usually painless and do not obstruct the epididymal tubule from which they arise. Resection of the spermatocele may cause epididymal obstruction.
Spermatocelectomy is indicated when associated with unremitting pain or when the spermatocele has grown to an uncomfortably large size. By definition, spermatoceles contain sperm. Puncture with a 30-gauge needle and identification of sperm in the aspirated fluid confirms the diagnosis. Stagnant sperm within a spermatocele occasionally leads to antisperm antibody formation and excision may eliminate these antibodies.
Vasoepididymostomy
Microsurgical approaches allow accurate approximation of the vasal mucosa to that of a single epididymal tubule41 resulting in marked improvement in the patency and pregnancy rates.4, 40, 42 Microsurgical vasoepididymostomy, however, is the most technically demanding procedure in all of microsurgery. In virtually no other operation are results so dependent on technical perfection.
Microsurgical vasoepididymostomy should only be attempted by experienced microsurgeons who perform the procedure frequently.
Indications
The indications for vasoepididymostomy at the time of vasectomy reversal are reviewed. For obstructive azoospermia not caused by vasectomy, vasoepididymostomy is indicated when the testis biopsy reveals complete spermatogenesis and scrotal exploration reveals the absence of sperm in the vasal lumen with no vasal or ejaculatory duct obstruction. The preoperative evaluation is identical to that described for vasovasostomy.
Microsurgical End-to-Side Intussusception Technique
This method, also known as the triangulation technique,42 has gained increasing popularity among microsurgeons over the conventional end-to-end and end-to-side vasoepididymostomy.4, 43, 44, 45 When the level of epididymal obstruction is clearly demarcated by the presence of markedly dilated tubules proximally and collapsed tubules distally, the site at which the anastomosis should be performed is readily apparent (Figs. 19 and 20).
The vas deferens is drawn through an opening in the tunica vaginalis and secured in proximity to the potential anastomotic site in the epididymis with two to four interrupted sutures of 6-0 polypropylene placed through the vasal adventitia and the epididymal tunica (Fig. 21). Six microdots are placed on the cut surface of the vas in an identical fashion to that described for vasovasostomy (Fig. 22). Three 10-0 double-armed nylon sutures are placed in the epididymal tubule in a triangular fashion (Fig. 23). The needles are not pulled through but left in situ, creating a triangle of needles. A generous opening is made in the epididymal tubule in the center of the triangle created by the three needles. The three needles are then pulled through (Fig. 24).
A glass slide is touched to the fluid exuding from the opening in the epididymal tubule and mixed with human tubal fluid media, covered with a cover slip and examined by the surgeon using the separate bench microscope under 400-power magnification. If sperm are present (whether motile or not), the decision is made to proceed with the anastomosis. Sperm are aspirated into micropipettes first (Fig. 25) and expressed into human tubal fluid media and sent for cryopreservation if motility is observed.
After abundant sperm have been aspirated into micropipettes and cryopreserved, the six needles are passed inside to out the vas deferens exiting through the six previously placed microdots in the order indicated (Fig. 26). Each pair of sutures is then sequentially tied. Tying of these sutures intussuscepts the epididymal tubule into the vas lumen (Fig. 27).
This creates a water-tight closure. In addition, the flow of epididymal fluid from the epididymal tubule into the vas deferens tends to plaster the edges of the epididymal tubule against the mucosal walls of the vas deferens, further helping to create a leakproof closure. The second layer of the anastomosis is completed using interrupted 9-0 nylon sutures to secure the epididymal tunica to the vasal sheath (Fig. 28).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Two-Stitch Longitudinal Variation of the Intussusception Technique
A two-stitch longitudinal variation of the intussusception technique has been described (Fig. 29).46 The two-stitch intussusception technique is much easier to perform, with a superior success rate.46, 47 For anastomoses to very small epididymal tubules such as those found in the caput or to the efferent ductules,44 the three-stitch triangular technique may be impossible.
This technique is currently our preferred method for all vasoepididymostomies. With this method, four microdots are marked on the cut surface of the vas deferens and two parallel sutures are placed in the distended epididymal tubule but not pulled through. After the fluid is tested for sperm and aspirated into micropipettes for cryopreservation, the four needles are passed inside-out the vas deferens and tied. The anastomosis is completed as described previously.
|
|
Long-Term Follow-Up Evaluation and Results
Microsurgical vasoepididymostomy in the hands of experienced and skilled microsurgeons will result in the appearance of sperm in the ejaculate in 50–85% of men. In a recent retrospective analysis of 47 patients,4 a patency rate of 65% was achieved at 1 month and 84% at 1 year postoperatively with the intussusception technique. Other smaller series have confirmed patency rates exceeding 80%.42, 45, 46 Pregnancy rates are higher the more distal in the epididymis the anastomosis is performed.
With the older vasoepididymostomy techniques at 14 months after surgery, 25% of initially patent anastomoses have shut down.1 For this reason, we recommend banking sperm both intraoperatively48 and as soon as they appear in the ejaculate postoperatively. In men with very low counts or poor sperm quality postoperatively and men who remain azoospermic, the sperm intraoperatively cryopreserved can be used for IVF with intracytoplasmic sperm injection.
Persistently azoospermic men without cryopreserved sperm can opt for either another vasoepididymostomy and/or microscopic epididymal sperm aspiration combined with IVF and intracytoplasmic sperm injection.
EJACULATORY DUCT OBSTRUCTION AND TRANSURETHRAL RESECTION OF THE EJACULATORY DUCTS
Ejaculatory duct obstruction is usually a congenital anomaly in which an aplastic segment occurs at the terminal end of the vas, where the ejaculatory duct enters the urethra; it is potentially correctable by transurethral resection.49 Occasionally, ejaculatory duct obstruction results from chronic prostatitis or extrinsic compression of the ejaculatory ducts by prostate or seminal vesical duct cysts.50
Diagnosis
Ejaculatory duct obstruction is suspected in azoospermic or severely oligo- and/or asthenospermic men with at least one palpable vas deferens, a low semen volume, acid semen pH, and negative, equivocal, or low semen fructose levels. If these men have normal serum levels of FSH and testis biopsy reveals normal spermatogenesis, the diagnosis of ejaculatory duct obstruction is entertained.
Digital rectal examination may reveal a midline cystic structure. Transrectal sonography has revolutionized the diagnosis and treatment of ejaculatory duct obstruction. A midline cystic lesion or dilated ejaculatory ducts and seminal vesicles can be visualized sonographically.
Transrectal ultrasound-guided aspiration of the cystic or dilated ejaculatory ducts or seminal vesicles can be performed.51 The aspirate is examined microscopically and if motile sperm are found, they can be cryopreserved and 2–3 mL of indigo carmine diluted with water-soluble radiographic contrast instilled. If an x-ray confirms a potentially resectable lesion (Fig. 30), then transurethral resection of ejaculatory ducts (TURED) is performed without the need for previous vasography as the presence of sperm in the seminal vesicles indicates that at least one epididymis is patent and that the cyst or dilated ejaculatory duct communicates with a nonobstructed vas.
The instillation of indigo carmine assists in localizing the opening of the ejaculatory duct and confirms when resection has successfully opened the obstructed system. Transrectal sonography with aspiration should be performed immediately before anticipated surgery and uses bowel preparation and antibiotic prophylaxis.
|
|
If no sperm are found in the aspirate, vasography is necessary. If no sperm are found in either vas and vasography reveals ejaculatory duct obstruction, coexisting bilateral epididymal obstruction is likely present. Simultaneous vasoepididymostomy and TURED has a low success rate. In this setting, it is best to abandon attempts at reconstruction and simply perform microsurgical epididymal sperm aspiration and cryopreservation for future IVF/ICSI.
Transurethral Resection of Ejaculatory Ducts Technique
A transurethral resectoscope, with a 24-French cutting loop, is engaged with a finger placed in the rectum providing anterior displacement of the posterior lobe of the prostate. The ejaculatory ducts course between the bladder neck and the verumontanum and exit at the level of and along the lateral aspect of the verumontanum (Fig. 31). Resection of the verumontanum of the prostate will often reveal the dilated ejaculatory duct orifice or cyst cavity (see Fig. 31).
Resection should be performed in this region with great care to preserve the bladder neck proximally, the striated sphincter distally, and the rectal mucosa posteriorly. Efflux of indigo carmine from dilated orifices confirms adequate resection. Avoid excessive coagulation. A Foley catheter is left overnight and the patient receives an additional 7 days of oral antibiotics.
|
|
Complications
Reflux
Reflux of urine into the ejaculatory ducts, vas, and seminal vesicles occurs after a majority of resections. This can be documented by voiding cystourethography or measuring semen creatinine levels.52, 53 Contamination of semen by urine impairs sperm quality.
Epididymitis
Reflux can lead to acute and chronic epididymitis. Recurrent epididymitis often results in epididymal obstruction. The incidence of epididymitis after TURED is probably underestimated. Symptomatic chemical epididymitis may occur from refluxing urine. Chronic low-dose antibacterial suppression, such as that used for vesico-ureteral reflux, may be necessary until pregnancy is achieved. If epididymitis is chronic and recurrent, vasectomy or even epididymectomy may be necessary.
Retrograde Ejaculation
Even when care has been taken to spare the bladder neck, retrograde ejaculation is common after TURED. Sudafed 120 mg orally, 90 minutes before ejaculation, may reverse this. If this is not successful, sperm can be retrieved from alkalinized urine and used for either intrauterine insemination or IVF with ICSI.
Results
TURED results in increased semen volume approximately two thirds of the time and appearance of sperm in the ejaculate in approximately 50% of previously azoospermic men. Pregnancy rates are based on case reports and small series.54 If viable sperm appear in the ejaculate but the quality is poor, IVF with ICSI is recommended, which currently yields delivery rates of up to 38.5% per attempt.
Because of the potential for serious complications, TURED should only be performed in azoospermic men or in severely oligoasthenospermic men and only after the couple has stated they are unwilling to perform IVF and have been fully apprised of the risks of TURED.
ELECTROEJACULATION
Men with neurological impairments in sympathetic outflow, such as seen in traumatic spinal cord injury, demyelinating neuropathies (multiple sclerosis), diabetes, and after retroperitoneal lymph node dissection, frequently have abnormalities in or absence of seminal emission. Ejaculation can be induced in most of these men, especially those with high spinal cord injury, with vibratory stimulation.55, 56, 57, 58 For those men who do not respond to vibratory stimulation, electroejaculation has proven to be a safe and effective means of obtaining motile sperm suitable for assisted reproduction techniques (IUI, IVF/ICSI).
The procedure is performed under general anesthesia, except for men with a complete spinal cord injury who do not require anesthesia. In men with a high thoracic spinal cord lesion (above T6) or in those men with previous history of autonomic dysreflexia, pretreatment, 15 minutes before the procedure, with 20 mg of sublingual nifedipine is used.
These men should have intravenous access and their blood pressure and pulse monitored every 2 minutes before, during, and for 20 minutes after electroejaculation. In the event of a sympathetic outflow (autonomic dysreflexia), termination of the procedure should be sufficient to break the response; however, intravenous access allows for delivery of sympatheticolytic agents should they become necessary.
Before placing the patient in the lateral decubitus position, the bladder is catheterized and emptied. A 12-French or 14-French silastic catheter lubricated with a small amount of mineral oil is used because commonly used lubricants are spermicidal. Ten milliliters of buffer (HEPES-BSA) is instilled into the bladder. Before the electroejaculation sequence, a digital rectal examination and anoscopy are performed. A rectal probe with three large horizontal stripes is well-lubricated, inserted with the electrodes facing anteriorly, and applied against the posterior aspect of the prostate and seminal vesicles.
The probe is connected to a variable output power source, which simultaneously records probe temperature through a thermistor in the rectal probe. Electrostimulation is started at 3–5 volts and increased in 1-volt increments with each stimulation. An assistant records probe temperatures, number of stimulations to full erection and ejaculation, and collects the ejaculate in a sterile wide mouth plastic container. The number of stimulations and maximum voltage required are variable and the ejaculate may be retrograde. If probe temperature rises rapidly or above 40°C, stimulation is suspended until the temperature decreases to below 38°C, or probes are changed.
At the completion of stimulation, anoscopy is again performed to check for rectal injury. The bladder is recatheterized to obtain any retrograde ejaculated sperm. The specimens are then delivered to the laboratory for processing. A second EEJ sequence can be immediately performed under the same anesthetic to obtain additional sperm.
Using this technique, sperm can be recovered in more than 90% of men. Overall pregnancy rates of up to 40% can be achieved after multiple cycles with intrauterine insemination. Use of IVF with ICSI will yield 50% live delivery rates for a single (albeit costly) procedure if motile sperm are obtained.
SPERM RETRIEVAL TECHNIQUES
Men with congenital absence or bilateral partial aplasia of vas deferens, or those with failed or surgically unreconstructable obstructions, can be treated by using sperm retrieval techniques in conjunction with IVF (Table 2).59, 60, 61
These techniques are also useful for intraoperative retrieval of sperm during reconstructive procedures such as vasoepididymostomy, which have significant failure rates. The intraoperatively retrieved sperm may be used immediately if the wife has been prepared for IVF, or may be cryopreserved for a future IVF with ICSI cycle, in the event the reconstructive surgery is unsuccessful. Sperm obtained from chronically obstructed systems usually have poor motility and decreased fertilizing capacity. The use of ICSI combined with IVF is essential to achieve optimal results.
Table 2. Comparison of the common methods used for the treatment of varicoceles
| Percutaneous Occlusion | Surgical Repair | ||||||
| Retrograde | Anterograde | Laparoscopic | Open retroperitoneal/high ligation | Open inguinal ligation | Open subinguinal ligation | Microsurgical | |
| Unperformable rate | 8–30% | Low | 0–11% | Low | Low | ||
| Recurrent/Persistence rate | 3–11% | 5–9% | 6–15% | 10–45% | 0–2% | ||
| Risk of arterial/lymphatic injury | Low | Very low | Moderate | High | Very low | ||
| Complication rate | 9–30% | 3–8% | 8–12% | 5–30% | <5% | ||
| Procedure time (mins/side) | 30–60 | 10–15 | 30–80 | 20–40 | 25–60 | ||
| Comments |
Local anesthesia
High unperformable rate for right side
Cost depends on method used
Risks of radiation exposure, contrast allergy, venous thrombosis or embolus, coil migration
|
Local anesthesia
Lower complication and recurrence rate than retrograde occlusion
Risks of radiation exposure, contrast allergy, venous thrombosis or embolus, coil migration
|
High cost
General anesthesia required
Risks of injury to viscera, peritonitis, arterial injury, lymphatic injury air embolism penumothorax subcutaneous emphysema deep vein thrombosis pneumoscrotum
|
Regional or general anesthesia required
High risks of arterial injury leading to testis atrophy and lymphatic injury leading to hydrocele
High recurrence/persistence rate because of missed venous returns
|
Local, regional, or general anesthesia
Lowest complication and recurrence rate
|
||
| References | 62, 63, 64, 65, 66, 67, 68, 69 | 70, 71, 72 | 69, 73, 74, 75, 76 | 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 | 5, 25, 83, 84, 85, 86 | ||
Microsurgical Epididymal Sperm Aspiration Techniques: Open Tubule Technique
The technique described here can be used for either intraoperative sperm retrieval at the time of vasoepididymostomy or as an isolated procedure in men with congenital absence of the vas or unreconstructable obstructions.48, 88 Under 16–25 times magnification using the operating microscope, the epididymal tunica is incised over a dilated tubule, which is isolated and incised with a 15-degree microknife. The fluid is touched to a slide, a drop of saline or Ringer’s is added, a coverslip is placed, and the fluid examined. If no sperm are obtained, the epididymal tubule and tunica are closed with 10-0 and 9-0 monofilament nylon sutures, respectively, and an incision is made more proximally in the epididymis or even at the level of the efferent ductules until motile sperm are obtained.
As soon as motile sperm are found, a dry micropipette (5 µL; Drummond Scientific, Broomall, Pennsylvania, USA) is placed adjacent to the effluxing epididymal tubule (Fig. 32). A standard hematocrit pipette is less satisfactory but can be used if micropipettes are not available. Sperm are drawn into the micropipette by simple capillary action.
Negative pressure, as is generated by action of an inline syringe, should not be applied during sperm recovery, because this may disrupt the delicate epididymal mucosa. Two micropipettes may be used simultaneously to increase speed of sperm recovery.
|
|
The highest rate of flow is observed immediately after incision of the tubule. Progressively better quality sperm are often found after the initial washout. Gentle compression of the testis and epididymis enhances flow from the incised tubule. With patience, 10–20 µL of epididymal fluid can be recovered.
The micropipette is connected to a short (3–5 cm) segment of medical-grade silicone tubing (American Scientific Products, McGaw Park, Illinois, USA). Alternatively, the tubing attached to a 22-gauge butterfly needle may be used. A 20-gauge needle fitted to a Luer-tip syringe is then placed in line. The fluid is flushed with IVF media (0.5–1.0 mL) into a sterile container. Once a micropipette has been used, it is discarded. Residual fluid in the pipette will disrupt capillary action. A typical procedure requires 4–12 micropipettes.
The sperm bank should be instructed to cryopreserve the aspirate in multiple vials (aliquots) so that several IVF cycles may be attempted if required.89
Experience with the technique has revealed that, paradoxically, in obstructed systems, sperm motility is better more proximally in the epididymis with the most motile sperm often found in the efferent ductules (Fig. 33). Motility immediately after aspiration and, consequently, fertilization rates, are highest in men who have the longest length of epididymal tubule available. Even when packed with debris distally, the epididymal tubule may be capable of secreting substances which can diffuse proximally and benefit sperm motility and fertilizing capacity.
|
|
Using ICSI, ongoing pregnancy or delivery rates exceeding 60% have been achieved with this technique using either fresh or cryopreserved epididymal sperm.38, 88 Epididymal sperm aspiration can be performed electively, with the cryopreserved sperm used for multiple future IVF cycles.89
Percutaneous Epididymal Sperm Aspiration
Percutaneous puncture of the epididymis with a fine needle (Fig. 34) has been successfully used to obtain sperm and achieve pregnancies.90, 91 The technique is less reliable than the open retrieval and the small quantities of sperm obtained are sometimes inadequate for cryopreservation. Reported pregnancy rates are half those achieved with open techniques. In view of the enormous costs and effort involved in IVF, epididymal sperm retrieval under direct vision is the preferred technique.
|
|
Testicular Sperm Extraction and Aspiration
The indications for testicular sperm extraction are failure to find sperm in the epididymis in the presence of the spermatogenesis or complete absence of the epididymis and nonobstructive azoospermia.7, 8, 92, 93
Testicular sperm have been retrieved employing one of three techniques: (1) open testicular sperm extraction, which allows retrieval of the largest number of sperm with potential for cryopreservation; this is the best technique in men with nonobstructive azoospermia; (2) percutaneous core biopsy, which uses the same 14-gauge biopsy gun used for prostate biopsy (Fig. 35); (3) percutaneous aspiration (testicular sperm aspiration [TESA]) with a high-suction glass syringe and a 23-gauge needle. This is the least invasive but requires 10–20 passes to obtain an adequate yield (Fig. 36).
|
|
|
|
Microsurgical Testicular Sperm Extraction
Testicular sperm extraction can result in injury to the testicular blood supply and lead to subsequent atrophy.94 The use of an operating microscope for standard open diagnostic testes biopsy allows identification of an area in the tunica albuginea free of blood vessels (Fig. 37), minimizing the risk of injury to testicular blood supply and allowing a relatively blood-free biopsy specimen.16 Using the microscope for testis biopsy, Schlegel15 discovered that in men with nonobstructive azoospermia, some of the tubules were larger than others.
The larger tubules are more likely to yield sperm. Previous studies95 revealed that testicular biopsy in men with nonobstructive azoospermia display considerable heterogeneity. Examination of permanently fixed biopsy specimens that display heterogeneity reveal that tubules with spermatogenesis are of considerably larger diameter than tubules that are Sertoli cell-only (Figs. 38 and 39). This difference can be readily observed under the operating microscope.
The percutaneous methods are most appropriate in men with normal spermatogenesis and obstructive azoospermia in whom adequate numbers of sperm can be retrieved in a small amount of tissue.32
|
|
|
|
|
|
Results
Using the microdissection technique, sperm have been identified in 50% of men explored. Pregnancy rates of 50% have been achieved with those men in whom sperm are found, at Cornell, using IVF/ICSI. The spontaneous abortion rate is 19%. The high rate of spontaneous abortion is probably because of the increased incidence of chromosomal abnormalities and DNA damage in the sperm of men with nonobstructive azoospermia.96
Even in severe cases of congenital or acquired testicular failure, as in Sertoli cell-only syndrome,93 postchemotherapy azoospermia,97 and nonmosaic (47,XXY) Klinefelter syndrome,98 sperm have been found and pregnancy and live births have been achieved.
IN VITRO FERTILIZATION WITH INTRACYTOPLASMIC SPERM INJECTION
IVF combined with the assisted fertilization technique of ICSI has enabled a highly successful treatment of severe male factor infertility.99, 100 It must be emphasized, however, that the availability of assisted reproductive technology does not obviate the need of proper work-up to diagnose the etiology of infertility and to provide a proper and specific treatment to reverse the underlying cause of infertility.
Rather, these techniques offer the opportunity for urologists to treat men who, just 10 years ago, would not have been candidates for any therapeutic interventions to father biological children. Examples of these cases, as discussed earlier in this chapter, include men with surgically unreconstructable obstruction such as congenital absence of the vas deferens; men with few viable sperm in the ejaculate; azoospermic men with varicoceles. Half of these men will respond to varicocelectomy with return of enough sperm to ejaculate to achieve pregnancy using IVF with ICSI.5, 25
The incidence of major fetal abnormalities is 3.2% compared with 1.6% in natural conception. IVF with ICSI is now the treatment of choice for severe uncorrectable male factor infertility. ICSI is costly ($12,000–15,000 for a single attempt) and usually not covered by most health insurance.
It also involves putting the female partner through a month of intramuscular injections, frequent sonographic monitoring, and measurement of blood hormone levels culminating in the transvaginal retrieval of multiple oocytes under sedation or general anesthesia. Aside from the cost and discomfort, each attempt is an emotional roller coaster for the couples. These procedures should not be applied indiscriminately. Every attempt should be made to treat the male and enable the couple to conceive as naturally as possible.
Most couples prefer natural conception. Specific treatment for male-factor infertility, such as microsurgical vasovasotomy, with a better than 50% natural pregnancy rate, and varicocelectomy with a natural pregnancy rate of 40–70% are more cost-effective than IVF/ICSI11, 12, 13, 14 and should be the first level of therapy.
VARICOCELECTOMY
Varicocele is by far the most commonly performed operation for the treatment of male infertility. Dilation of venous pampiniform plexus is secondary to defective or absence of valves in the internal spermatic veins. This is especially common on the left side, where the internal spermatic vein drains directly into the renal vein.
Dilatation of the venous structures results in impaired counter current heat exchange and increased testicular temperature. Testicular temperature is elevated by almost 2°C in men with varicoceles compared with controls.101 Both spermatogenesis and Leydig cell function for hormonal production are impaired at this elevated temperature. In addition, poor venous drainage may lead also to hypoxia and increased testicular pressure that may be detrimental to normal testicular function.
Finally, elevated spermatic venous catecholamines, possibly from adrenal reflux, may contribute to the negative impacts of varicoceles on testicular function.
Varicocele is found in approximately 15% of the general population, 35% of men with primary infertility, and in 70–81% of men with secondary infertility.23, 102 Animal and human studies have demonstrated that varicocele is associated with a progressive and duration-dependent decline in testicular function.17, 18, 19, 20, 22, 23, 102, 103, 104
A careful physical examination is the key to diagnose varicoceles. Before the examination, the patient should be relaxed. If the examination room is cold, a heating pad can be used to keep the scrotum warm and relaxed to allow a proper examination.
In grade I varicocele, an impulse can be palpated in the scrotum during a Valsalva maneuver. A grade II varicocele is large enough for tortuous and dilated veins to be palpated without a Valsalva maneuver. Grade III varicocele is visible through the scrotal skin. Color Doppler ultrasound can be used to confirm the diagnosis. Typically, when more than two to three veins of 3 mm or greater in size were found, with enlargement on standing and reflux on Valsalva maneuver, the diagnosis is made. Varicoceles that are impalpable on a good physical examination are considered subclinical and probably do not warrant treatment.104
Repair of varicocele will halt any further damage to testicular function19 and, in a large percentage of men, result in improved spermatogenesis,105 as well as enhanced Leydig cell function.26Table 2 summarizes the pros and cons of various methods of varicocele repair.
Retroperitoneal Operations
Retroperitoneal repair of varicocele involves incision at the level of the internal inguinal ring (Fig. 40A), splitting of the external and internal oblique muscles (see Fig. 40B), and exposure of the internal spermatic artery and vein retroperitoneally near the ureter (see Fig. 40C).
This approach has the advantage of isolating the internal spermatic veins proximally, near the point of drainage into the left renal vein. At this level, only one or two large veins are present and, in addition, the testicular artery has not yet branched and is often distinctly separate from the internal spermatic veins. Retroperitoneal approaches involve ligation of the fewest number of veins. This approach is still a commonly employed method for the repair of varicocele, especially in children.
|
|
A disadvantage of a retroperitoneal approach is the high incidence of varicocele recurrence, especially in children and adolescents, when the testicular artery is intentionally preserved. Recurrence rates after retroperitoneal varicocelectomy are in the range of 15%.106, 107 Failure is usually caused by preservation of the periarterial plexus of fine veins (venae comitantes) along with the artery.
These veins have been shown to communicate with larger internal spermatic veins. If left intact, they may dilate with time and cause recurrence. Less commonly, failure is caused by the presence of parallel inguinal or retroperitoneal collaterals, which may exit the testis and bypass the ligated retroperitoneal veins rejoining the internal spermatic vein proximal to the site of ligation.108, 109 Dilated cremasteric veins, another cause of varicocele recurrence,110 cannot be identified with a retroperitoneal approach.
Positive identification and preservation of the 0.5–1.5 mm testicular artery via the retroperitoneal approach is difficult, especially in children in whom the artery is small. Because at this level the internal spermatic vessels cannot be delivered into the wound, the operation involves working in a deep hole to dissect and ligate the vessels in situ in the retroperitoneum. In addition, the difficulty in positively identifying and preserving lymphatics using this approach results in postoperative hydrocele formation in 7–33% of retroperitoneal operations.111 The incidence of recurrence appears to be higher in children, with rates reported between 15% and 45% in adolescents.112, 113, 114, 115, 116, 117 Kass118 reports that recurrence can be markedly reduced in children and adolescents by intentional ligation of the testicular artery. This assures ligation of the periarterial network of fine veins.
Although reversal of testicular growth failure has been documented with intentional testicular artery ligation at the time of retroperitoneal repair in children, the effect of artery ligation on subsequent spermatogenesis is uncertain. In adults, bilateral artery ligation has been documented to occasionally cause azoospermia and testicular atrophy. At the least, it is inarguable that testicular artery ligation will not enhance testicular function.
Laparoscopic Varicocelectomy
Laparoscopic repair is, in essence, a retroperitoneal approach and many of the advantages and disadvantages are similar to those of the open retroperitoneal approach.119, 120, 121, 122, 123, 124 Using the laparoscope, the internal spermatic vessels and vas deferens can be clearly visualized through the laparoscope as they course through the internal inguinal ring. The magnification provided by the laparoscope allows visualization of the testicular artery. With experience, the lymphatics may be visualized and preserved as well.
With laparoscopic varicocelectomy, the internal spermatic veins are ligated at the same level as the retroperitoneal (Palomo) approach described. Laparoscopic varicocelectomy should allow preservation of the testicular artery in a majority of cases, as well as preservation of lymphatics. The incidence of varicocele recurrence would be expected to be similar to that associated with the open retroperitoneal operations. As in open retroperitoneal operation, these recurrences would be caused by collaterals joining the internal spermatic vein near its entrance to the renal vein or entering the renal vein separately.
Currently, reported series of laparoscopic varicocelectomy are too small and the follow-up interval too short to determine the incidence of recurrence and complications. The potential complications of laparoscopic varicocelectomy (injury to bowel, vessels or viscera, air embolism, peritonitis) are significantly more serious than those associated with the open techniques. Furthermore laparoscopic varicocelectomy requires a general anesthetic.
The microsurgical techniques described next can be performed using local or regional anesthesia and employ an incision of 2.5–3 cm for unilateral repair. This is equal to or less than the sum of incisions used for a laparoscopic approach. Furthermore, postoperative pain and recovery from the laparoscopic technique are the same as those associated with subinguinal varicocelectomy.120 Finally, laparoscopic varicocelectomy takes at least twice as long to perform and is less cost-effective than open varicocelectomy. Only in the hands of an experienced laparoscopist may the approach be considered a reasonable alternative for the repair of bilateral varicoceles.122
Microsurgical Inguinal and Subinguinal Operations: The Preferred Approaches
Inguinal and subinguinal varicocelectomy are currently the most popular approaches (Fig. 41). They have the advantage of allowing the spermatic cord structures to be pulled up and out of the wound so that the testicular artery, lymphatics, and small periarterial veins may be more easily identified. In addition, an inguinal or subinguinal approach allows access to external spermatic and even gubernacular veins, which may bypass the spermatic cord and result in recurrence if not ligated.
Finally, an inguinal or subinguinal approach allows access to the testis for biopsy or examination of the epididymis for obstruction.
|
|
Traditional approaches to inguinal varicocelectomy involve a 5–10 cm incision made over the inguinal canal, opening of the external oblique aponeurosis, and encirclement and delivery of the spermatic cord. The cord is then dissected and all the internal spermatic veins are ligated.115 The vas deferens and its vessels are preserved. An attempt is made to identify and preserve the testicular artery and, if possible, the lymphatics.
In addition, the cord is elevated, and any external spermatic veins that are running parallel to the spermatic cord or perforating the floor of the inguinal canal are identified and ligated. Compared with retroperitoneal operations, conventional nonmagnified inguinal approaches lower the incidence of varicocele recurrence but do not alter the incidence of either hydrocele formation or testicular artery injury. Conventional inguinal operations are associated with an incidence of postoperative hydrocele formation varying from 3 to 15% with an average incidence of 7%.111
Analysis of the hydrocele fluid has clearly indicated that hydrocele formation after varicocelectomy is caused by ligation of the lymphatics.111 The incidence of testicular artery injury during nonmagnified inguinal varicocelectomy is unknown. Case reports, however, suggest that this complication may be more common than realized. It can result in testicular atrophy and, if the operation is performed bilaterally, azoospermia may ensue in a previously oligospermic man. Furthermore, Starzl and his transplant group125 reported a 14% incidence of testicular atrophy and 70% incidence of hydrocele formation when the spermatic cord was divided and only the vas and vasal vessels preserved.
The introduction of microsurgical technique to varicocelectomy (Fig. 42) has resulted in a substantial reduction in the incidence of hydrocele formation.5, 126 This is because the lymphatics can be more easily identified and preserved (Fig. 43). Furthermore, the use of magnification enhances the ability to identify and preserve the 0.5–1.5 mm testicular artery (Fig. 44), thus avoiding the complications of atrophy or azoospermia.
|
|
|
|
|
|
Advocates of nonmicrosurgical techniques contend that the deferential (vasal) artery and, if preserved, the cremasteric artery, will provide adequate blood supply to the testes to prevent atrophy. However, anatomic studies have shown that the diameter of the testicular artery is greater than the diameter of the deferential artery and cremasteric artery combined. The testicular artery is the main blood supply to the testes. At the very least, it is inarguable that ligation of the testicular artery is unlikely to enhance testicular function.
Radiographic Occlusion Techniques
Intraoperative venography has been used to visualize the venous collaterals that, if left unligated, may result in varicocele recurrence.108, 113, 127, 128 Intraoperative venography does reduce the incidence of varicocele recurrence, but the two-dimensional view afforded often does not enable the surgeon to identify the location of all collaterals.
Percutaneous procedures for varicoceles include the traditional retrograde occlusion129, 130, 131 and the more recently described anterograde technique.62, 63, 87 In the retrograde technique, which can be performed under a local anesthetic, the right femoral vein is punctured to insert an angiocatheter to gain access to the internal spermatic vein via the inferior vena cava and the left renal vein. On confirming the anatomy and the presence of reflux in the testicular vein, it is occluded in a retrograde fashion (against the natural direction of the internal spermatic venous return).
Percutaneous occlusion is a suitable treatment option for persistent/recurrent varicoceles postsurgical repair.64 The use of imaging techniques to identify the cause of varicocele recurrence allows accurate venous occlusion while eliminating the need for a difficult dissection of the fibrous adhesions from previous surgery.
Inexpensive sclerosing agents are commonly used for retrograde occlusion. Newer embolization techniques, using more expensive materials such as detachable coils65, 66 and occlusive balloons,131 have been described.
Complications, including contrast reaction, flank pain, migration of embolizing materials, infection, thrombophlebitis, arterial puncture, and hydroceles, occur at a significant rate (9–30%).87, 99, 124, 125, 126, 127, 128, 130, 131, 132, 133, 134 In addition, the radiographic techniques take between 1 and 3 hours to perform compared with 25–45 minutes required for surgical repair.
Venographic placement of a balloon or coil in the internal spermatic vein is successfully accomplished in 75–90% of attempts;132, 133, 134 therefore, a significant number of men undergoing attempted radiographic occlusion will ultimately require a surgical approach. Unsuccessful cases are commonly seen in right-side varicoceles because of venous anatomical variations and difficulties in gaining proper venous access. Hence percutaneous retrograde varicocele occlusion is best used for isolated left-side varicoceles.
The recurrence rate after balloon occlusion was originally 11% but later reportedly as low as 4%.87, 99, 124, 125, 126, 127, 128, 130, 131, 132, 133, 134, 135, 136 Failure to successfully cannulate small collaterals and external spermatic veins results in recurrence.
Percutaneous anterograde varicocele occlusion, by injection of sclerosing agents into an isolated vein from the pampiniform plexus in the scrotum after confirming its drainage fluoroscopically (Fig. 45), has been described.62, 63, 87 As with the retrograde procedure, anterograde occlusion can be performed under local anesthesia. Furthermore, the anterograde technique is associated with a lower operating time (10–15 minutes).
Although the complication rate is only 3–8%, testicular atrophy post-treatment, likely secondary to unidentified arterial injury, has been reported in 1% of cases.87 While the persistence/recurrence rate for large varicoceles can be as high as 25%, the overall figure in short-term follow-up is 5–9%. Long-term follow-up, however, is not available and the consequence of escape of the sclerosing agent into the renal vein and vena cava is unknown.
|
|
Complications of Varicocelectomy
Hydrocele formation is the most common complication reported after nonmicroscopic varicocelectomy. The incidence of this complication varies from 3 to 33%, with an average incidence of approximately 7%. Analysis of the protein concentration of hydrocele fluid indicates that hydrocele formation after varicocelectomy is caused by lymphatic obstruction.111
At least half of postvaricocelectomy hydroceles grow to a size large enough to warrant surgical excision caused by the discomfort and growth of the hydrocele to a large size. The effect of hydrocele formation on sperm function and fertility is uncertain. It is known that men with varicocele have significantly elevated intratesticular temperatures,101, 135, 136 and this appears to be an important pathophysiological phenomenon mediating the adverse effects of varicocele on fertility. The development of a large hydrocele creates an abnormal insulating layer that surrounds the testis. This may impair the efficiency of the countercurrent heat exchange mechanism and therefore obviate some of the benefits of varicocelectomy.
Use of magnification to identify and preserve lymphatics can virtually eliminate the risk of hydrocele formation after varicocelectomy.126, 137, 138 Also, radiographic balloon or coil occlusion techniques eliminate the risk of hydrocele formation.
Testicular Artery Injury
The diameter of the testicular artery in humans is 0.5–1.5 mm (see Fig. 44). Microdissections of the human spermatic cord have revealed that the testicular artery is closely adherent to a large internal spermatic vein in 40% of men. In another 20% of men, the testicular artery is surrounded by a network of tiny veins.139 During the course of cord dissection for varicocelectomy, the artery may go into spasm and even in its unconstricted state is often difficult to positively identify and preserve.
Injury or ligation of the testicular artery carries with it the risk of testicular atrophy and/or impaired spermatogenesis. Starzl’s transplant group125 reported a 14% incidence of frank testicular atrophy when the testicular artery was purposely ligated. The actual incidence of testicular artery ligation during varicocelectomy is unknown, but some studies suggest it is common.140 Animal studies indicate that the risk of testicular atrophy after testicular artery ligation varies from 20 to 100%.30, 141
In humans, atrophy after artery ligation is probably less likely because of the contribution of the cremasteric as well as vasal arterial supply. In children, the potential for neovascularization and compensatory hypertrophy of the vasal and cremasteric vessels is probably greater than in adults, making atrophy after testicular artery ligation less likely. Use of magnifying loupes, or preferably an operating microscope and/or a fine-tipped Doppler probe, facilitates identification and preservation of the testicular artery and therefore minimizes the risk of testicular injury.142 Radiographic balloon or coil occlusion techniques also eliminate this risk.
Varicocele Recurrence
The incidence of varicocele recurrence after surgical repair varies from 0.6 to 45%. Recurrence is more common after repair of pediatric varicoceles. Radiographic studies of recurrent varicoceles visualize periarterial, parallel inguinal or mid-retroperitoneal collaterals, or, more rarely, transcrotal collaterals.72
Retroperitoneal operations miss parallel inguinal collaterals. Nonmagnified inguinal operations have a lower incidence of varicocele recurrence than retroperitoneal operations but fail to address the issue of scrotal collaterals or small veins surrounding the testicular artery. The microsurgical approach with delivery of the testis lowers the incidence of varicocele recurrence to 1–2%, compared with 9–16% using nonmagnified inguinal techniques.126, 137, 138
Results
Varicocelectomy results in significant improvement in semen analysis in 60–80% of men. Reported pregnancy rates after varicocelectomy vary from 20 to 60%. A randomized controlled trial of surgery versus no surgery in infertile men with varicoceles revealed a pregnancy rate of 44% at 1 year in the surgery group versus 10% in the control group.143 In our series of 1500 microsurgical operations, 43% of couples were pregnant at 1 year137 and 69% at 2 years when couples with female factors were excluded. Microsurgical varicocelectomy results in return of sperm to the ejaculate in 50% of azoospermic men with palpable varicoceles.5, 25
The results of varicocelectomy are also related to the size of the varicocele. Repair of large varicoceles results in a significantly greater improvement in semen quality than repair of small varicoceles103, 144 (Fig. 46).
In addition, large varicoceles are associated with greater preoperative impairment in semen quality than small varicoceles, and consequently overall pregnancy rates are similar regardless of varicocele size. In the presence of small (grade I) varicoceles along with larger (grade II and III) contralateral ones, greater improvement in semen parameters can be expected if repair is performed bilaterally than if only the larger side is repaired.145 Some evidence suggests that the younger the patient is at the time of varicocele repair, the greater the improvement after repair and the more likely the testis is to recover from varicocele induced injury.21
Varicocele recurrence, testicular artery ligation, or postvaricocelectomy hydrocele formation are often associated with poor postoperative results. In infertile men with low-serum testosterone levels, microsurgical varicocelectomy alone results in substantial improvement in serum testosterone levels.26
|
|
Summary
Varicocele is an extremely common entity, present in 15% of the male population. Varicoceles are found in approximately 35% of men with primary infertility but 75–81% of men with secondary infertility. Mounting evidence clearly demonstrates that varicocele causes progressive duration-dependent injury to the testis. Larger varicoceles appear to cause more damage than small varicoceles and, conversely, repair of large varicoceles results in greater improvement of semen quality. Varicocelectomy can halt the progressive duration-dependent decline in semen quality found in men with varicoceles.
The earlier the age at which varicocele is repaired, the more likely is recovery of spermatogenic function. Variococelectomy can also improve Leydig cell function resulting in increased testosterone levels.26
The most common complications after varicocelectomy are hydrocele formation, testicular artery injury, and varicocele persistence or recurrence. The incidence of these complications can be reduced by using microsurgical techniques, with inguinal or subinguinal operations, and exposure of the external spermatic and scrotal veins. Use of these advanced techniques of varicocelectomy provide a safe effective approach to elimination of varicocele, preservation of testicular function, and, in a substantial number of men, an increase in semen quality and likelihood of pregnancy.
ORCHIOPEXY IN ADULTS
Cryptorchidism is the condition when the testis fails to descend into its normal scrotal location postnatally. Embryologically, the testis develops in the abdominal region. Thus, its descent may be inhibited anywhere along its normal pathway from intra-abdomen to the inguinal canal. It is well-known that cryptorchidism is associated with a high incidence of infertility even when unilateral.
Long hot baths and saunas in humans, on a regular basis, have been shown to impair spermatogenesis. Elevated testicular temperature is also thought to be the primary pathophysiologic feature of varicocele.101, 135, 136, 146 Spermatogenesis is exquisitely temperature-sensitive. Both animal and human studies have shown that artificial elevation of testicular temperature results in impaired spermatogenesis. In pigs, testes that are pushed into the abdomen and secured there will stop making sperm.147 Orchiopexy restores spermatogenesis.148, 149
In pigs with surgically created cryptorchid testes, cooling of the testes with an intra-abdominal cooling device restores spermatogenesis.150
Undescended Testis in Adults
An undescended testis sometimes escapes detection until adulthood. If the contralateral testis is normal, these men may be fertile. If both testes are truly undescended, azoospermia is certain. In the past, bilateral orchiectomy was usually recommended in adults with undescended testes because of the substantially increased risk of testes tumor. Orchiectomy, however, condemns these men to absolute sterility and a lifetime of hormone replacement therapy.
Bilateral orchiopexy in adults can result in induction of spermatogenesis and pregnancy.148 It will also preserve testicular hormonal function. The technique of orchiopexy in adults is identical to that used for children. Even with a normal contralateral testis, orchiopexy is worthwhile to bring down a unilateral undescended testis to, if possible, a scrotal location where it can be examined.
Leydig cell function in undescended testis can be retained. Even a solitary cryptorchid testis, when properly placed in scrotum, can provide enough testosterone to obviate the need for hormone replacement. Although the goal of orchiopexy in adults is to achieve a scrotal testis, an inguinal or subcutaneous location that permits examination is acceptable. When orchiopexy is performed in adults, regular self-examination and yearly sonography is mandatory.
Retractile Testes in Adults
Retractile testes are different from cryptorchid testes. Retractile testes are the high-riding testes that can be manually manipulated to stay down into the scrotum, either in the office or under anesthesia. In boys, they are usually not surgically repaired and they almost always fully descend into the scrotum as the testes grow, particularly at puberty. The fate of persistently retractile testis in adults is unknown.
A subset of infertile men have retractile testis.151 The semen analyses of these men often demonstrate a typical stressed pattern similar to those of men with varicoceles. These men, however, do not have palpable varicoceles. They all have at least one and frequently both testes that retract out of the scrotum and into the abdomen and remain there for 1 hour or more per day. In some men, these testes remain in the abdomen virtually all the time, except when in a warm shower or under anesthesia.
It is likely that these testes will have impaired temperature regulation and impaired spermatogenesis. Scrotal orchiopexy can improve the semen quality and fertility of some of these men.
Authors

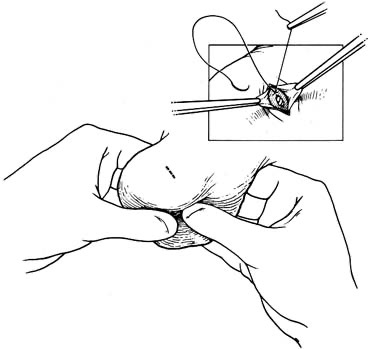 Fig. 1. A 0.5 cm transverse incision over upper lateral or
Fig. 1. A 0.5 cm transverse incision over upper lateral or  Fig. 2. A 3–4 mm incision is made on the tunica albuginea, avoiding major crossing vessels.
Fig. 2. A 3–4 mm incision is made on the tunica albuginea, avoiding major crossing vessels. Fig. 3. Sampling of seminiferous tubules using razor-sharp iris scissors.
Fig. 3. Sampling of seminiferous tubules using razor-sharp iris scissors. Fig. 4. A touch-prep is made by multiple blotting on the cut seminiferous tubules with a glass slide.
Fig. 4. A touch-prep is made by multiple blotting on the cut seminiferous tubules with a glass slide. Fig. 5. Intraoperative wet prep reveals sperm with tails (solid arrows). Immature germ cells are seen as well (open arrows).
Fig. 5. Intraoperative wet prep reveals sperm with tails (solid arrows). Immature germ cells are seen as well (open arrows). Fig. 6. A squash-prep is made by crushing the seminiferous tubules on a slide.
Fig. 6. A squash-prep is made by crushing the seminiferous tubules on a slide. Fig. 7. Closure of the tunica albuginea with interrupted 6-0 nylon.
Fig. 7. Closure of the tunica albuginea with interrupted 6-0 nylon. Fig. 8. Preferred incisions for vasectomy reversal allow extension to external inguinal ring (marked by x) when abdominal vas is short.
Fig. 8. Preferred incisions for vasectomy reversal allow extension to external inguinal ring (marked by x) when abdominal vas is short.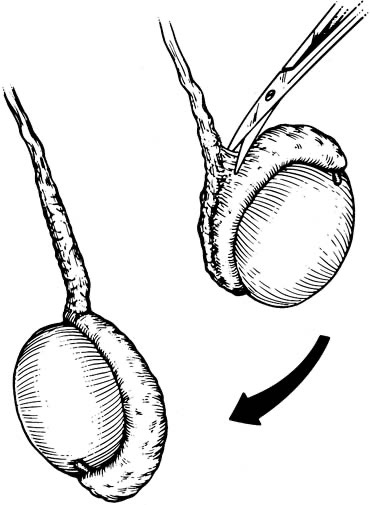 Fig. 9. Convoluted vas dissected off the epididymal tunica provides additional length on testicular side.
Fig. 9. Convoluted vas dissected off the epididymal tunica provides additional length on testicular side. Fig. 10. Ultrasharp knife drawn through a 2–3 mm slotted nerveholding clamp (from Accurate Surgical and Scientific Instrument Corp, Westbury, New York, USA) produces a perfect 90 degree cut.
Fig. 10. Ultrasharp knife drawn through a 2–3 mm slotted nerveholding clamp (from Accurate Surgical and Scientific Instrument Corp, Westbury, New York, USA) produces a perfect 90 degree cut. Fig. 11. Inside-out placement of a 10-0 mucosal suture.
Fig. 11. Inside-out placement of a 10-0 mucosal suture. Fig. 12. Precision placement of sutures is facilitated by using a microtip marking pen to map out planned needle exit points. Lines are drawn at 3 o’clock and 9 o’clock to help match them up. This mapping prevents dog-ears and leaks when the lumen diameters are discrepant.
Fig. 12. Precision placement of sutures is facilitated by using a microtip marking pen to map out planned needle exit points. Lines are drawn at 3 o’clock and 9 o’clock to help match them up. This mapping prevents dog-ears and leaks when the lumen diameters are discrepant. Fig. 13. First layer: Placement of a total of six 10-0 mucosal sutures. Note that all sutures exit through microdots.
Fig. 13. First layer: Placement of a total of six 10-0 mucosal sutures. Note that all sutures exit through microdots. Fig. 14. Second layer. Deep mucularis sutures placed exactly between the mucosal sutures.
Fig. 14. Second layer. Deep mucularis sutures placed exactly between the mucosal sutures. Fig. 15. Second layer. The 9-0 sutures placed just above but not through the mucosa to seal gaps between mucosal sutures.
Fig. 15. Second layer. The 9-0 sutures placed just above but not through the mucosa to seal gaps between mucosal sutures. Fig. 16. Completed second layer.
Fig. 16. Completed second layer. Fig. 17. Third layer. The 9-0 sutures placed on the adventitial layer.
Fig. 17. Third layer. The 9-0 sutures placed on the adventitial layer. Fig. 18. Fourth layer. Approximation of vasal sheath with 7-0 PDS.
Fig. 18. Fourth layer. Approximation of vasal sheath with 7-0 PDS.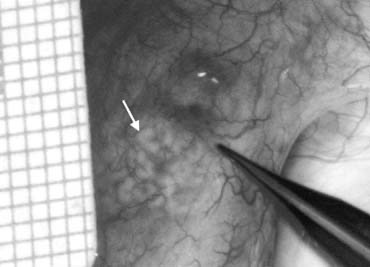 Fig. 19. Inspection of the epididymis for dilated tubules (arrow) seen beneath the epididymal tunica.
Fig. 19. Inspection of the epididymis for dilated tubules (arrow) seen beneath the epididymal tunica.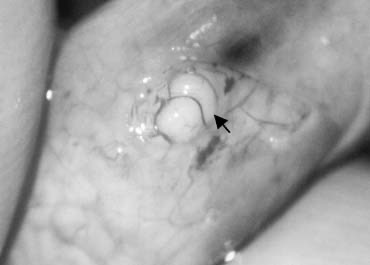 Fig. 20. Dissection exposing dilated loops of epididymal tubule (arrow).
Fig. 20. Dissection exposing dilated loops of epididymal tubule (arrow). Fig. 21. Preparation for vasoepididymostomy. The 6-0 sutures approximate the posterior lip of vasal adventitia to lower edge of opening tailored in the epididymal tunica.
Fig. 21. Preparation for vasoepididymostomy. The 6-0 sutures approximate the posterior lip of vasal adventitia to lower edge of opening tailored in the epididymal tunica. Fig. 22. As in vasovasosotomy, the use of microdots on the cut surface of the vas can enhance precision in suture placement.
Fig. 22. As in vasovasosotomy, the use of microdots on the cut surface of the vas can enhance precision in suture placement. Fig. 23. Three 10-0 nylon sutures are placed on the epididymal tubule in a triangulation fashion. To avoid leaking of fluid, which will lead to collapse of the tubule and difficult placement of subsequent sutures, the needles are left in place. In addition, accidental cutting of the sutures is avoided when opening the tubule with a sharp microknife.
Fig. 23. Three 10-0 nylon sutures are placed on the epididymal tubule in a triangulation fashion. To avoid leaking of fluid, which will lead to collapse of the tubule and difficult placement of subsequent sutures, the needles are left in place. In addition, accidental cutting of the sutures is avoided when opening the tubule with a sharp microknife.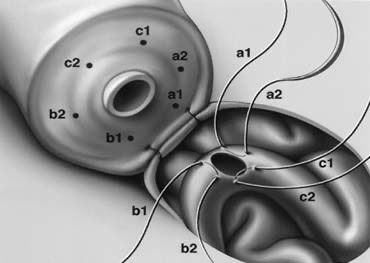 Fig. 24. Position of sutures in the epididymal tubule once the tubule is opened.
Fig. 24. Position of sutures in the epididymal tubule once the tubule is opened. Fig. 25. Once the presence of sperm in the epididymal tubule has been confirmed, epididymal fluid is aspirated by capillary action into micropipette for cryopreservation.
Fig. 25. Once the presence of sperm in the epididymal tubule has been confirmed, epididymal fluid is aspirated by capillary action into micropipette for cryopreservation. Fig. 26. The six needles are passed inside-out the vas deferens exiting through the six microdots.
Fig. 26. The six needles are passed inside-out the vas deferens exiting through the six microdots. Fig. 27. Tying the sutures intussuscepts the epididymal tubule into the vas lumen.
Fig. 27. Tying the sutures intussuscepts the epididymal tubule into the vas lumen. Fig. 28. Closure of the second layer with 9-0 sutures.
Fig. 28. Closure of the second layer with 9-0 sutures. Fig. 29. Two-needle longitudinal intussusception vasoepididymostomy. The longitudinal placement of needles allows for a longer tubular incision, resulting in a larger opening.
Fig. 29. Two-needle longitudinal intussusception vasoepididymostomy. The longitudinal placement of needles allows for a longer tubular incision, resulting in a larger opening.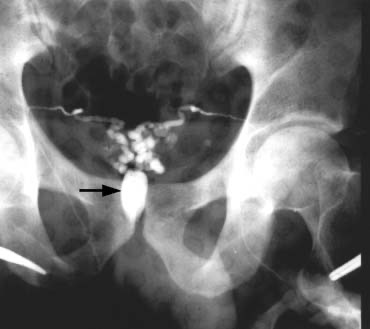 Fig. 30. Both vasa are visualized after injection of contrast into only the right vas. The vasa empty into a common cavity, likely a mid-line ejaculatory duct cyst (arrow).
Fig. 30. Both vasa are visualized after injection of contrast into only the right vas. The vasa empty into a common cavity, likely a mid-line ejaculatory duct cyst (arrow). Fig. 31. Location of the ejaculatory ducts (ED) in relation to the prostatic urethra (PU). Obstruction of the ejaculatory ducts can be managed with transurethral resection.
Fig. 31. Location of the ejaculatory ducts (ED) in relation to the prostatic urethra (PU). Obstruction of the ejaculatory ducts can be managed with transurethral resection. Fig. 32. Sperm drawn into micropipette by capillary action.
Fig. 32. Sperm drawn into micropipette by capillary action. Fig. 33. In obstructed epididymis, more motile sperm can be found in
Fig. 33. In obstructed epididymis, more motile sperm can be found in 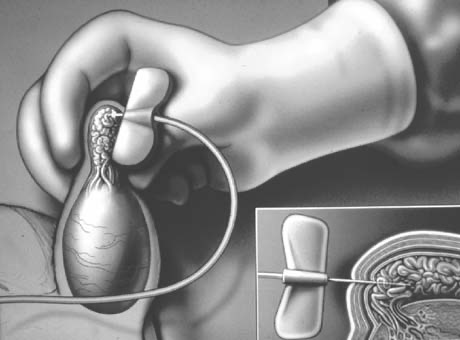 Fig. 34. Percutaneous epididymal sperm aspiration (PESA).
Fig. 34. Percutaneous epididymal sperm aspiration (PESA). Fig. 35. Percutaneous core biopsy for sperm retrieval.
Fig. 35. Percutaneous core biopsy for sperm retrieval. Fig. 36. Testicular sperm aspiration (TESA).
Fig. 36. Testicular sperm aspiration (TESA).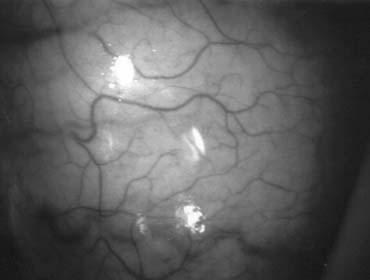 Fig. 37. Microsurgical techniques allow clear identification of blood vessels on the tunica albuginea of the testis.
Fig. 37. Microsurgical techniques allow clear identification of blood vessels on the tunica albuginea of the testis.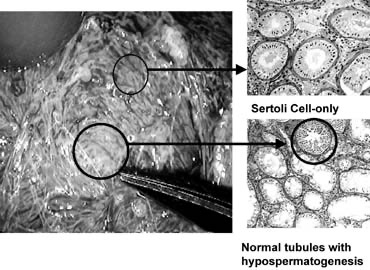 Fig. 38. Tubules with spermatogenesis are of larger diameter than tubules with Sertoli cell-only pattern.
Fig. 38. Tubules with spermatogenesis are of larger diameter than tubules with Sertoli cell-only pattern.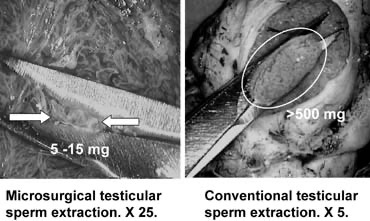 Fig. 39. Selective removal of large seminiferous tubules with microsurgical testicular sperm extraction. A significantly smaller quantity of tissue is taken compared with conventional nonmicrosurgical testicular sperm extraction.
Fig. 39. Selective removal of large seminiferous tubules with microsurgical testicular sperm extraction. A significantly smaller quantity of tissue is taken compared with conventional nonmicrosurgical testicular sperm extraction.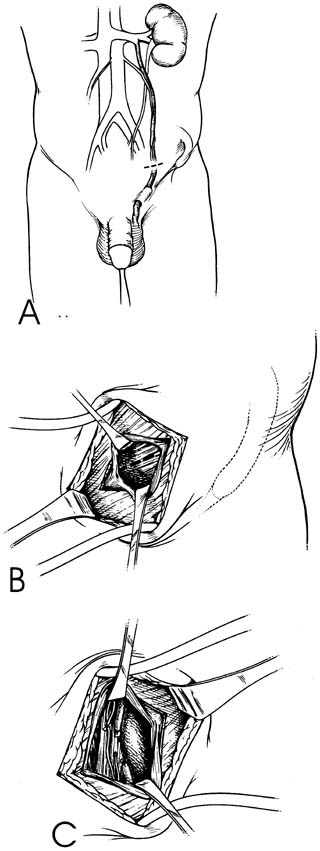 Fig. 40. Retroperitoneal varicocelectomy.
Fig. 40. Retroperitoneal varicocelectomy. Fig. 41. Inguinal incision beginning at the external inguinal ring (X) and extending 3 cm laterally along the skin lines. Subinguinal incision just below the ring.
Fig. 41. Inguinal incision beginning at the external inguinal ring (X) and extending 3 cm laterally along the skin lines. Subinguinal incision just below the ring.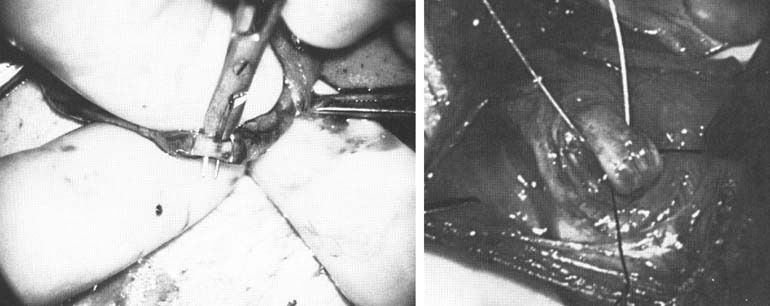 Fig. 42. Internal spermatic veins are cleaned and ligated with either hemoclips or double 4-0 silks, one black and one white, passed beneath them before ligation and division.
Fig. 42. Internal spermatic veins are cleaned and ligated with either hemoclips or double 4-0 silks, one black and one white, passed beneath them before ligation and division.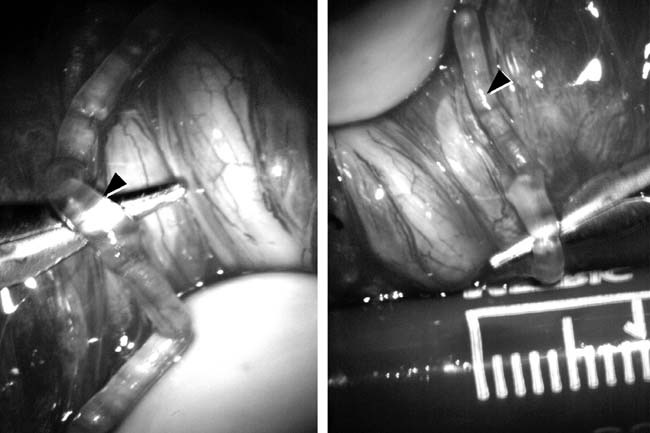 Fig. 43. Lymphatics (arrowheads) are clearly identified and preserved.
Fig. 43. Lymphatics (arrowheads) are clearly identified and preserved. Fig. 44. The testicular artery is identified and tagged with a vessel loop.
Fig. 44. The testicular artery is identified and tagged with a vessel loop.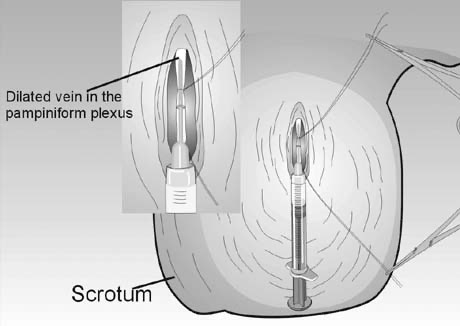 Fig. 45. Percutaneous anterograde varicocele occlusion. A dilated vein from the pampiniform plexus of the spermatic cord is dissected and cannulated for injection of sclerosing agent for occlusion.
Fig. 45. Percutaneous anterograde varicocele occlusion. A dilated vein from the pampiniform plexus of the spermatic cord is dissected and cannulated for injection of sclerosing agent for occlusion.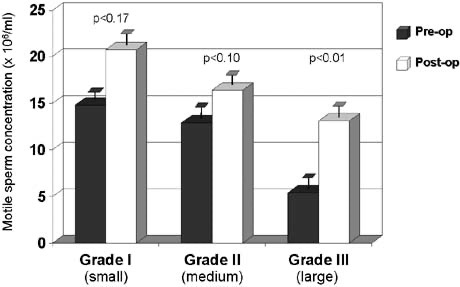 Fig. 46. Relationship between varicocele size and response to repair.
Fig. 46. Relationship between varicocele size and response to repair.