In a surprising reversal of its earlier stand, US recently announced that it would back a plan to waive Covid-19 vaccine patents, in an attempt to boost production of shots needed to inoculate most of the world.
The talks on the waiver of intellectual property rights for COVID-19 vaccines is yet to begin at World Trade Organization (WTO), but, likely to face stiff opposition from some member countries.
The plan was originally mooted by India and South Africa in Oct 2020 at the WTO, arguing that an IP waiver for Covid-19 vaccines could help resolve the urgent issues blocking access to these products. They asked WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement for the waiver to remain in place until most of the world has been vaccinated against Covid-19.
It has to be acknowledged that there is a huge divide between the developed and developing nations with regard to fair, equitable and affordable access to Covid-19 products, including vaccines and drugs. Rich countries have signed bilateral deals with the vaccine manufacturers to secure vaccines in advance while the developing nations are in the end of the queue. It is this inequality and inequity caused by affordability and availability that has resulted in the proposal from the said two countries.
The initial IP waiver proposal by India and South Africa last October included vaccines, treatments, diagnostic kits, ventilators, protective gear and other products needed to battle the COVID-19 pandemic.
“Some countries are racing to vaccinate their entire populations while other countries have nothing.”
This gap “is growing every day and becoming more grotesque every day.”
-@DrTedros pic.twitter.com/xfyXzIH3N6
— Global Health Strategies (@GHS) May 15, 2021
While the proposal had many backers from the developing nations, it did not get the support from the developed countries.
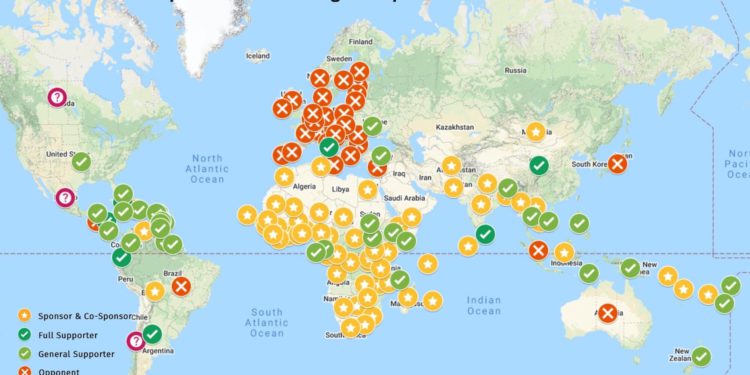
World Health Organization (WHO) welcomed the move of US, as its attempt last year to promote the pooling of intellectual property — the Covid-19 Technology Access Pool — only received lukewarm response from countries and companies holding the IP rights.
Unsurprisingly, pharmaceutical industry has also voiced its opposition to the IP waiver of vaccines.
The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) said, “Diluting national and international IP frameworks during this pandemic is counterproductive… IP enables research and development and ensures that the next generation of inventors and investors will remain engaged.”
Thomas Cueni, director-general of IFPMA, felt that the waiver alone would not help people get inoculated fast across the world. He succinctly put it this way, saying, “You could get the recipe from Mary Berry for the loveliest cake you can imagine. But if you try to replicate that cake, good luck.”
Médecins Sans Frontières (MSF) , which operates in many low-income countries, wholehearted welcomed the IP waiver move of the States. “This monumental decision will help address the historic and extraordinary global health challenges we’re facing and increase equitable access to lifesaving COVID-19 vaccines worldwide by helping to end this crisis for everyone. The longer it takes to vaccinate everyone in the world, the greater the risk to us all as new variants have more opportunity to take hold.”
”While this decision means other manufacturers will have the information they need from pharmaceutical corporations—and the legal permission—to help scale up global supply and get more shots into the arms of people everywhere, this won’t happen immediately.”
MSF added, “If the US truly wants to end this pandemic, it must also share its surplus vaccines doses with COVAX now and fill the access gap until additional manufactures are able to scale up production.
The WHO is also trying to encourage vaccine manufacturers to share their patents and technical knowhow with low-income countries. There are also appeals from activists to ease export controls, so vaccine makers are not restricted in where they send their shots, or the raw materials for the vaccine.
Prashant Yadav, a supply chain expert at the Center for Global Development, said patents were unlikely to help in the next six months because of tight supplies but they could be useful to help set up a manufacturing network that could come online in 12 to 18 months.
“Vaccines, unlike drugs, cannot be made with just a patent waiver. It has to be followed up with tech transfer, especially with new technology like mRNA vaccines, which are not easy to manufacture.”
-@doctorsoumya pic.twitter.com/ZUSYTU6nvv
— Global Health Strategies (@GHS) May 15, 2021
The IP waiver of vaccines has enthused many governments and drug manufacturers, but, a realistic look at the current stage of negotiations would only indicate that a dramatic increase in the availability of vaccines appears remote this year.
(source: https://www.pharmaceutical-technology.com/features/wto-ip-waiver-proposal-covid19-vaccine/
2. https://www.msf.org/countries-obstructing-covid-19-patent-waiver-must-allow-negotiations
3. Top image source: msf.org| TADEU ANDRE/MSF)